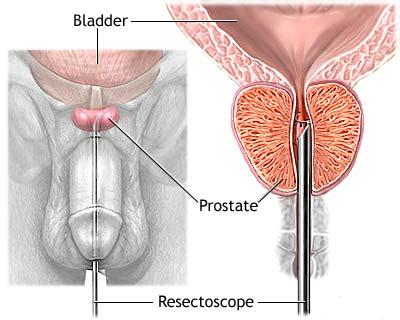
Prostate cancer marker (psa) (ug/l)
Conținutul
Lancet Oncol ; 20 8 : This phase trial evaluated the safety prostate cancer marker (psa) (ug/l) efficacy of the hafnium oxide HfO2 nanoparticle NBTXR3 activated by radiotherapy versus radiotherapy alone as a pre-operative treatment in patients with locally advanced soft-tissue sarcoma. Sarc is a phase randomised, multicentre, international trial.
Patients had to have a WHO performance status of and a life expectancy of at least 6 months. Randomisation was stratified by histological subtype myxoid liposarcoma vs others.
This was an open-label study.

The primary endpoint was the proportion of patients with a pathological complete response, assessed by a central pathology review board following European Organisation for Research and Treatment of Cancer guidelines in the intention-to-treat population full analysis set. Safety analyses were done in all patients who received at least one puncture and injection of NBTXR3 or at least one dose of radiotherapy.
This study is registered with ClinicalTrials.
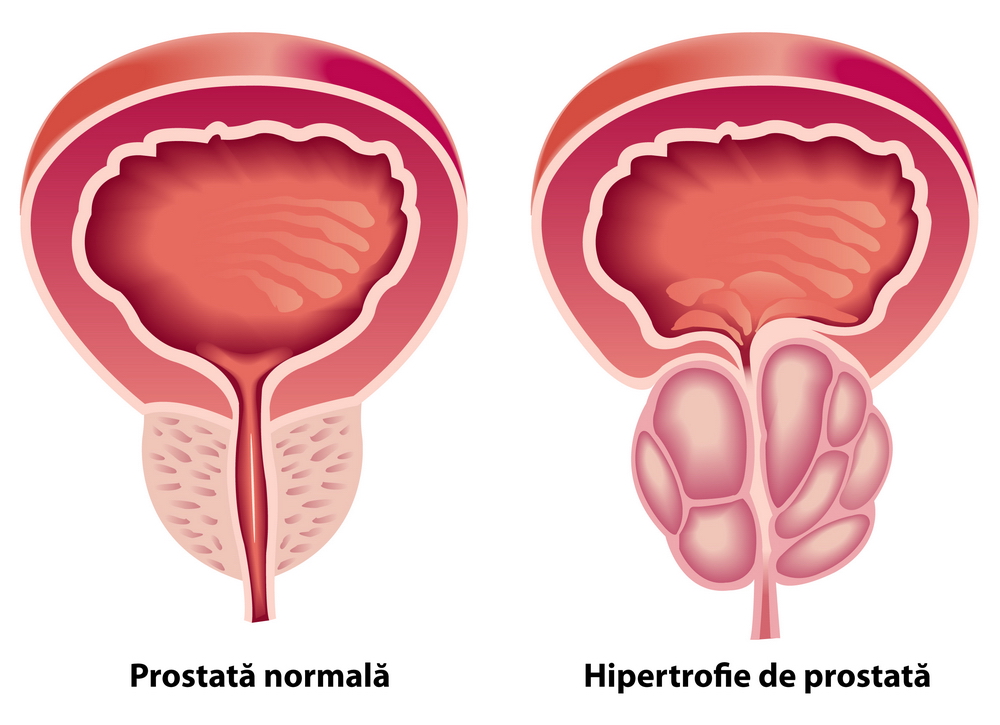
Trestioreanu Bucureşti Rezumat În ultimii 10 ani, cel mai utilizat marker tumoral pentru detecţia cancerului de prostată a fost antigenul spectfic prostatic PSA. Concentraţia de PSA total din ser este proporţională cu volumul prostatei la pacienţii cu cancer de prostată, fiind un indicator foarte sensibil al modificărilor care apar la aceştia.
Two patients in the NBTXR3 group and one patient in the radiotherapy group were excluded from the efficacy analysis because they were subsequently discovered to be ineligible; thus, a total of patients were analysed for the primary endpoint in the intention-to-treat full analysis set 87 in the NBTXR3 group and 89 in the radiotherapy alone group. No treatment-related deaths occurred.